Biological Molecules and its Types, Concepts of Biology
Biological Molecules
Organic molecules (also called biomolecules) are the basic building blocks of living cells. Biological Molecules are small and large organic molecules. For example, water is a small organic molecule made up of two types of atoms (oxygen and hydrogen).
Large molecules are called biological macromolecules, four of which are biologically important. The class of biological molecules includes DNA and RNA. In this article, we mainly focus on large molecules, so the term biological macromolecule will be used in components.
The large molecules necessary for microbial life are called macromolecules. There are four major classes of biological macromolecules (carbohydrates, lipids, proteins, and nucleic acids), each of which is an integral part of the cell and performs many functions.
Together, these molecules make up the bulk of the cell. Biological macromolecules contain organic, i.e. carbon (except carbon dioxide). In addition, they may contain hydrogen, oxygen, nitrogen, phosphorus, sulfur, and other trace elements.
Elements of Biological Molecules
Carbon
It is often said that life is “carbon-based”. This means that carbon atoms, linked to other carbon atoms or other elements, are the major components of many, if not most, of the unique molecules for living things. Other elements play important roles in biological molecules, but carbon certainly qualifies as the “primary” element for molecules in living things. These are the bonding properties of carbon atoms that are responsible for their important role.
Carbon Bonding
Carbon has four electrons in its outer shell. Thus, it can form four covalent bonds with other atoms or molecules. The simplest organic carbon molecule is methane (CH4), which consists of four hydrogen atoms associated with one carbon atom.
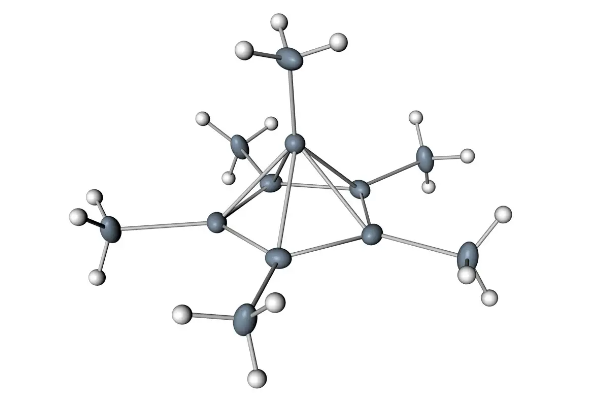
However, more complex systems are made with carbon. Any hydrogen atom can be replaced with another carbon atom associated with the first carbon atom. In this way, long carbon chains and branches can be created (Figure 2a). Carbon atoms can bond to atoms containing other elements, such as nitrogen, oxygen, and phosphorus (Figure 2b). The molecule can also form rings, which can be fused with other rings (Figure 2c). This type of molecule explains the type of function of biological macromolecules and is mainly based on the ability of carbon to form many bonds with itself and other atoms.
Also Read:
- Nephrology and Nephrology Education and its related Diseases
- Greenhouse Gas its Effects and Greenhouse Gases emissions
- Effects of Acid Rain, Its Most Important Effects on Human
Carbohydrates
Carbohydrates are macromolecules that are well known to consumers. Some people go on a “low carb” diet to lose weight. On the other hand, athletes often load up on carbs before important competitions to ensure they have enough energy to compete at a high level. In fact, carbohydrates are an important part of our diet; Whole grains, fruits, and vegetables are natural sources of carbohydrates. Carbohydrates provide energy to the body, primarily through glucose, a simple sugar. Carbohydrates play vital roles in humans, animals, and plants.
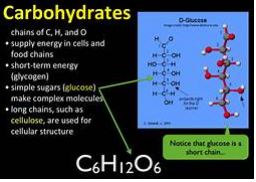
Carbohydrates can be expressed by the formula (CH2O)n, where n is the number of carbon atoms in the molecule. In other words, the ratio of carbon to hydrogen to oxygen in a carbohydrate molecule is 1:2:1. Carbohydrates are divided into monosaccharides, disaccharides, and polysaccharides.
Monosaccharides
Monosaccharides (mono- = “one”; sacchar- = “sweet”) are simple sugars, the most common of which is glucose. In monosaccharides, the number of carbon atoms usually ranges from three to six. Most monosaccharide names end with the suffix -ose. Depending on the number of carbon atoms in the sugar, they may be known as trioses (three carbon atoms), pentoses (five carbon atoms), and hexoses (six carbon atoms).
Monosaccharides may exist as a linear chain or as ring-shaped molecules; in aqueous solutions, they are usually found in the ring form.
The chemical formula for glucose is C6H12O6. In most living species, glucose is an important source of energy. During cellular respiration, energy is released from glucose, and that energy is used to help make adenosine triphosphate (ATP). Plants synthesize glucose using carbon dioxide and water by the process of photosynthesis, and glucose, in turn, is used for the energy requirements of the plant. The excess synthesized glucose is often stored as starch that is broken down by other organisms that feed on plants.
Galactose
Galactose (a component of lactose or milk sugar) and fructose (in fruits) are other common monosaccharides. Although the chemical model (C6H12O6) is similar to glucose, galactose and fructose, the structure and chemistry (known as an inmate) are different due to the structure of the atoms in the carbon chain.
Disaccharides
Disaccharides (di- = “two”) are formed when two monosaccharides undergo a dry reaction (a reaction to remove water atoms). In this process, the hydroxyl group (–OH) of one monosaccharide bonds to the hydrogen atom of another monosaccharide, releasing water atoms (H2O) and forming a covalent bond between the atoms in the atom, two sugars.
Common disaccharides are lactose, maltose and sucrose. Lactose is a disaccharide containing the monomers glucose and galactose. This only happens in milk. Maltose or malt sugar is a disaccharide formed by a dry reaction between two sugar atoms. The disaccharide is usually sucrose or table sugar, made from glucose and fructose monomers.
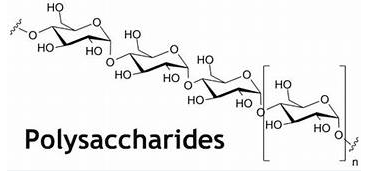
Polysaccharides
Polysaccharides (poly = “many”) are long chains of monosaccharides linked by covalent bonds. Chains can be branched or unbranched and contain a variety of monosaccharides. Polysaccharides can have a high molecular weight. Starch, glycogen, cellulose, and chitin are examples of polysaccharides.
Starch
Starch is a type of sugar stored in plants and contains amylose and amylopectin (both glucose polymers). Plants can produce glucose, and glucose levels are stored as hydro ethyl starch in various plant parts, including roots and seeds. Starch hydro ethylene fed to animals is broken down into smaller molecules like glucose. Cells can absorb glucose.
Glycogen
Glycogen is a storage form of glucose in humans and other vertebrates and contains glucose monomers. Glycogen is an animal that resembles hydroxyethyl starch and is a highly branched molecule that is normally stored in the liver and muscles. When glucose levels drop, glycogen breaks down and releases glucose.
Cellulose
Cellulose is a highly volatile organic compound. The wall of plant cells is made of cellulose, which supports the cells. Plants and leaves are mostly cellulose and natural. Cellulose is a collection of sugar monomers that bond carbon atoms to sugar atoms together.
Other cellulosic sugar monomers, such as long chains, rotate and fill well. This gives the strong cellulose a lot of strength needed to grow plants. It is called fiber and moves through the digestive tract. While food enzymes cannot break down cellulose-glucose bonds, herbivorous animals, such as cows, buffalo and horses, can break down cellulose residues for food.
These animals contain certain types of bacteria. In the rumen (part of the nest of plant cells) the enzyme secretes cellulase. The supplement also contains cellulose-breaking bacteria, which play an important role in the body’s digestive system. Cellulose can break down cellulose into glucose monomers that can be used by animals as an energy source.
Carbohydrates work differently in different animals. Species such as insects, spiders and spiders have an outer shell called an exoskeleton to protect their internal organs. This exoskeleton is a biological chromatic macromolecule, nitrogenous hydrocarbon. Many processed sugars contain nitrogen.
These can vary in molecular structure due to different roles played by carbohydrate energy release (starch and glycogen) and structural thickening and breakdown (cellulose and chitin).
Lipids
Lipids retain a diverse group of compounds closely related to common characteristics. Lipids are hydrophobic (“waterproof”) or insoluble in water because of their neutrally polar molecules. It came about that the carbon dioxide is with all the polar carbon-of carbon-water bonds. Some functions of lipids in the cell.
Cells reduce energy for long-term consumption as lipids are consumed. Lipids were required for the isolation of plants and their environment (Figure 5). For example, keeping waterfowl and mammals over waterways helps them have water-repellent properties. Lipids are both components of many hormones and a rich component of the plasma membrane. Lipids include vets, oils, waxes, phospholipids, and steroids.
Fats
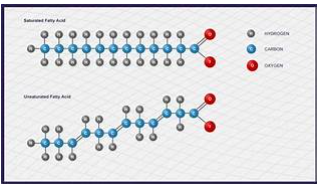
A fat molecule, like a triglyceride, consists of two main components: glycerol and fatty acids. Glycerin is an organic compound with three carbon atoms, five hydrogen atoms, and three hydroxyl groups (-OH). Fatty acids are long chains of carbohydrates with an acidic carboxyl group attached, hence the name “fatty acid”.
The number of carbon atoms in fatty acids can be from 4 to 36; Most have 12-18 carbon atoms. A fat molecule contains a fatty acid that is covalently bonded to each of the three oxygen atoms in the –OH groups of the glycerol molecule.
Phospholipids Type of Biological Molecules
These are the components of the plasma membrane. Like fat, it consists of a chain of fatty acids attached to a glycerol backbone or the like. However, there are two fatty acids, not three, and the third carbon in the glycerol backbone is bonded to a phosphate group. Modified by adding alcohol to the phosphate group
Steroids and Waxes
Unlike phospholipids and fats discussed earlier, the ring structure is given to steroids. Although they are not like other lipids, they cluster together because they are also hydrophobic. All steroids have four carbon rings and some, like cholesterol, have a short tail.
Cholesterol is a steroid. Cholesterol is mainly synthesized in the liver and is a precursor to many steroid hormones such as testosterone and estradiol. It is also a precursor to vitamins E and K. Cholesterol is a precursor to bile salts, which aid in the breakdown of fats and subsequent cellular absorption. Although we often talk about cholesterol in a negative way, it is necessary for the proper functioning of the body. It is an important part of the plasma membranes of animal cells.
Waxes consist of a hydrocarbon chain with an alcohol group (-OH) and a fatty acid. Examples of animal waxes are beeswax and lanolin. Plants have a waxy coating on their leaves that prevents them from drying out.
A phospholipid has both hydrophobic and hydrophilic regions. Fatty acid chains are hydrophobic and detached from water while phosphate is hydrophilic and reacts with water.
The cells are surrounded by a membrane made up of two layers of phospholipids. The fatty acids of phospholipids face inward away from water while the phosphate group faces outward or inward in the cell, both of which are aqueous.
Proteins
Proteins are among the most abundant organic molecules in living systems and perform the most diverse functions of any macromolecule. Proteins can be structural, regulatory, contractile, or protective; to be used for transport, storage or sheathing; or it could be toxins or enzymes. Every cell in a living system contains thousands of different proteins, each with a unique function. Their structure and functions are significantly different. However, they are all polymers of amino acids arranged in a linear order.
The functions of proteins are very diverse because there are 20 different chemically different amino acids that form long chains, and the amino acids can be arranged in any order. For example, proteins can act like enzymes or hormones. Enzymes produced by living cells catalyze biochemical reactions (such as digestion) and are usually proteins.

Each enzyme is specific for the substrate (enzyme binding reagent) on which it acts. Enzymes can break molecular bonds, repair bonds, or form new bonds. An example of an enzyme is salivary amylase, which breaks down amylose, a starch component.
Hormones are chemical signaling molecules, usually, proteins or steroids, secreted by an endocrine gland or group of endocrine cells that regulate or control certain physiological processes, including growth, development, metabolism, and reproduction. For example, insulin is a protein hormone that regulates blood sugar levels.
Proteins have different molecular shapes and masses; Some proteins are spherical in shape, while others are fibrous. For example, hemoglobin is a globular protein and collagen is a fibrous protein in our skin. The shape of proteins is crucial for their function.
Changes in temperature, pH, and chemical exposure can cause irreversible changes in protein form, resulting in loss of function or denaturation (discussed in more detail later). All proteins contain different arrangements of the same 20 different amino acids.
Amino acids are the monomers that makeup proteins. Each amino acid has the same basic structure, consisting of an amino group (-NH2), a carboxyl group (-COOH), and a central carbon atom linked to hydrogen atoms. Each amino acid has a different variable atom or group of atoms attached to the central carbon atom, called an R group, the R group is the only structural difference between the 20 amino acids; The rest of the amino acids are the same