Why is Ozone Layer Important? Environmental Sciences?
What is an Ozone Layer?
The ozone layer, also known as the ozonosphere, is a layer of the upper atmosphere that extends about 15 to 35 kilometers above the earth and contains large ozone layers (O3). About 90% of the ozone in the air is in the stratosphere. The Stratosphere extends from 10–18 km (6–11 mi) to about 50 km above sea level.
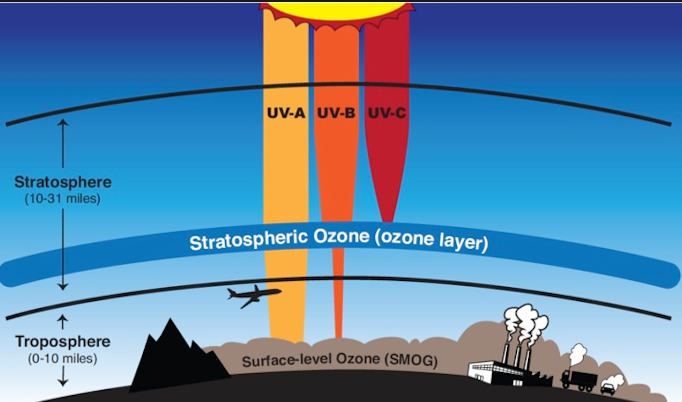
In the stratosphere, air temperature increases at a higher rate, resulting from the absorption of solar radiation by the ozone layer. The ozone layer effectively prevents solar radiation from reaching the earth below 290 nm. This includes ultraviolet (UV) radiation and other forms of radiation that can damage or kill many living things.
Also Read:
- Greenhouse Gas its Effects and Greenhouse Gases emissions
- Effects of Acid Rain, Its Most Important Effects on Human
- Watershed Management its Developmental Projects and its Significance
Location in Earth’s Atmosphere
It is located about 20 to 25 km from the center of the earth. Its peaks range from 26 to 28 km (about 16 to 17 centimeters) in the tropics and from 12 to 20 km in the tropics. The tree is visible. The upper altitude of the middle layer extends from the orbit to the lower layer of air between the upper atmosphere and the lower part of the Tropopause
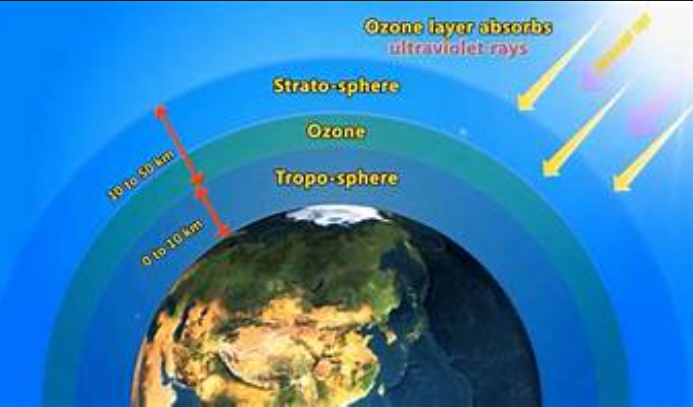
Most of the remaining ozone is found in the troposphere, in the air that flows into the stratosphere behind the Earth. Ozone depletion is often due to the interaction between certain pollutants (such as nitrogen oxides and volatile organic compounds), strong sunlight and hot weather is one of the main causes of photochemical smog, a problem faced by many cities and towns. From all over the world, especially in the summer months
Ozone creation and destruction
The formation of ozone in the stratosphere is mainly due to photons breaking the chemical bonds between high-energy oxygen molecules (O2) in the sun. A process called photolysis releases singlet oxygen atoms, which combine with free oxygen molecules to form ozone.
Almost two. Scientists say ozone layer formation is important for the evolution of life on Earth by eliminating deadly UVB radiation (ultraviolet rays with a wavelength of 315-280 nm) and facilitating the movement of life from Earth’s oceans. He played a leading role.
The amount of ozone in the stratosphere naturally changes throughout the year due to the chemical processes that create and destroy ozone molecules, as well as winds and other transport processes that move ozone molecules around the planet. However, over several decades, human activities have significantly altered the ozone layer.
Ozone depletion, the global decline in stratospheric ozone observed since the 1970s, is most striking in the Polar Regions and correlates well with increases in stratospheric chlorine and bromine. Once chlorofluorocarbons (CFCs) and other halocarbons (carbon-halogen compounds) are released by UV radiation, these chemicals destroy ozone by removing individual oxygen atoms from ozone molecules. The depletion is so strong that so-called ozone holes (areas with significantly reduced ozone cover) form over the poles at the beginning of the respective spring seasons. The largest such hole, covering more than 20.7 million square kilometers (8 million square miles) continuously since 1992, appears over Antarctica annually between September and November.
Benefits of Ozone Layer
- The ozone (O3) molecule consists of three oxygen atoms. It is an allotrope of oxygen. It is different from dioxide (O2), which we need to breathe.
- It is a light blue gas under normal conditions and a dark blue liquid at cryogenic temperatures. It is unstable and very reactive.
- An oxidizing agent more potent than molecular oxygen, At concentrations above 100 ppb, it is a serious health hazard and a pollutant near the Earth’s surface.
- Ozone near the surface of the earth is known as tropospheric ozone, tropospheric ozone and bad ozone. That’s 10% of all the ozone on Earth.
- What happened to the ozone layer hole? † Discovery magazine
- It is developed from two sources:-
- From the stratosphere to the troposphere ozone is transported
- Reactions between nitrox ides and volatile organic compounds (the result of human activities)
- In the stratosphere, it is known as stratospheric ozone and fine ozone.
- Ozone in the stratosphere makes up 90% of the ozone on Earth. In the stratosphere, ozone is formed as a result of a reaction between UV radiation and oxygen molecules.
- O2 + UV = O + O
- The resulting oxygen atoms are highly reactive. Each of them can react with a different dioxygen molecule to form an ozone molecule.
- For example, UV radiation energy is used to break down oxygen molecules and create the ozone layer.
- O2 + O = O3
- Good stratospheric ozone acts as a protective shield for us because it can absorb harmful ultraviolet radiation effectively, resulting in the following benefits:
- Protects against skin diseases
- Protecting plants from various diseases and indirectly helping them maintain biogeochemical cycles.
- Supporting the phytoplankton population in the marine ecosystem
- Protection of many marine animals in the early stages of their development
What Causes Ozone Layer Depletion?
When certain chemicals produced by industrial and human activities reach the atmosphere and are exposed to UV light, chlorine and bromine are released.
This chlorine and bromine begin to destroy ozone molecules.
It should be noted that the natural production of ozone is much slower than the depletion due to chemicals produced by human activities. Chlorine atoms can destroy thousands of ozone molecules.
Chemicals that can deplete the ozone layer are called ozone-depleting substances (ODS).
Pay close attention to the chemical composition of chlorine and bromine.
How to prevent Ozone depletion?
International organizations were concerned about protecting the ozone layer from the SDGs in the 1970s and 1980s.
The Vienna Convention on Ozone Protection establishes international cooperation for the protection of the stratospheric oceans. In 1987, 149 countries signed the Montreal Protocol.
Early reports after the signing of the Montreal Protocol indicate that the ozone layer is severely weakened. Thus, in 1992, as part of the protests, an amendment was made to the provisions of the original 1987 agreement to halt the production of Halen in 1994, and in 1996 to halt the production of CFCs in developed countries.
Importance of Ozone Layer
The ozone layer consists of two layers of the atmosphere. The first 10% of ozone is in the troposphere and the remaining 90% is in the stratosphere. There are different types of sunlight that hit the earth and UV rays are harmful to the human body. The ozone absorbs harmful UV rays and prevents them from reaching the surface. In addition, ozone in the lower atmosphere absorbs a variety of gases.
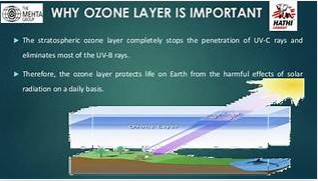
It is almost impossible to live without ozone because without ozone you can get many diseases like skin cancer, eye diseases, and so on. Harmful UV rays can also harm plants and marine life. A recent study found that 97% of an Australian city’s curse had disappeared, leading to a significant loss of skin patients in the country.
Conclusion
Based on the above reasoning, we can conclude that the ozone layer is a protective layer necessary for the survival of life on Earth. It absorbs 95 percent of the sun’s harmful UV rays. Thus, without ozone, we are more susceptible to many health problems.
Every year World Ozone Day is celebrated on 16 September. The day will be used to spread awareness about ozone depletion and its consequences.